
Ochre
In common with that other family of pigments dug out of the ground, the umbers, those that were designated ochres saw constant use in house-painting from the earliest times. Not only were they readily obtainable, but they encompassed a large range of hues, both in their natural and their calcined (roasted) state.
The Swiss chemist and author Pierre François Tingry (1743 – 1821) explained how readily they were obtained:
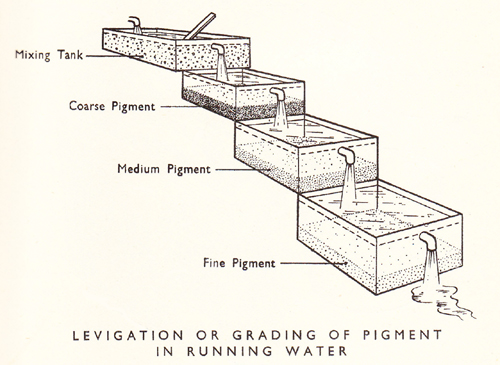
“Ochres are easily purified by simple washing. They mix readily with water, and the sand and stones which they contain being heavier than themselves, subside. The water, turbid with the ochre, is decanted, by making it pass into a trough lower than the vessel in which it was washed; when the ochre has subsided the clear water is drawn off. The ochre is then taken out, and being dried is divided into small masses”.1
In 1676, an English writer, in what was perhaps the first manual on house-painting, mentioned the two basic types:
“Yellow Oaker, Is of two sorts; the one gotten in England, the other brought from beyond the Seas: the one is light Yellow, much like the colour of Wheat straw; the other is somewhat of a deeper colour”.2
The second edition clarified this, by referring to the first as “Plain-Oaker” most of which was found in the Shotover Hills near Oxford, and the other as “Spruce-Oaker”. The former displayed many of the best properties for a house-painting pigment, being described as a -
“Colour, that with pains, will grind very fine, it bears an excellent body, and resists the weather well”.3
A darker ochre sometimes called “Common Brown or Bristol Oker” was recommended for filling imperfections in the body work of carriages, presumably a greater capacity for drying rendered it useful for this purpose.4
Essentially ochres are derived from sands and clays found in many parts of the world. The mixture of silica, alumina and hydrated iron oxide giving them their yellow colour. They are generally light- and lime-fast and also inert. However, being a natural product ochres can vary in both colour and quality in the same underground seam. This inconsistency led to them being synthesised from an early date. Many methods were used, but the principal one consisted of the oxidation of iron sulphate (also known as green vitriol or copperas mixed with alum and precipitated by means of an alkali.5
As well as various sorts of yellow, Professor Tingry told us that:
“Many of the yellow ochres when burnt become of a red colour, and are then occasionally used for more delicate processes”.6
When calcined the pigment was generally known as light red, as can be seen above. If further heat is applied the colour deepens to a dark purply-brown. This process was applied to both natural and synthetic yellow ochres. When the latter were involved the darkest version was known by the exotic name of Colcothar of Vitriol.

Oxford Ochre
The earliest detailed account of Oxford Ochre is given by Robert Plot, the first Professor of Chemistry at Oxford University. In his Natural History of Oxfordshire of 1677, he describes how difficult it was to find:
“They dig it now at Shotover on the East side of the hill, on the right hand of the way leading from Oxford to Whately [Wheatley], though questionless it may be had in many other parts of it; the vein dips from East to West, and lies from seven to thirty feet in depth, and between two and seven inches thick; enwrapped it is within ten folds of earth, all which must be past through before they come at it; for the earth is here, as at most other places, I think I may say of a bulbous nature, several folds of divers colours and consistencies, still including one another, not unlike the several coats of a Tulip root, or onyon.
The 1. next the turf, is a reddish earth
2. a pale blue clay
3. a yellow sand
4. a white clay
5. an iron stone
6. a white, and sometimes a reddish maum
7. a green, fat, oily kind of clay
8. a thin iron rubble, almost like Smiths cinders.
And then the Yellow Ochre, which is of two parts.
1. The Stone Ochre, which we may also call native, because ready for use as ’tis dug: and
2. Clay Ochre, which because of the natural inequality in its goodness, they wash and steep two or three days in water, and then beat it with clubs on a plank into thin broad cakes, of an equal mixture both of good and bad; then they cut it into squares like Tiles, and put it on hurdles laid on trestles to dry, which when thoroughly done ’tis fit for the merchant.”7
Pockets of ochre of varying descriptions were found throughout the area, the best being on the estate belonging to Shotover House. An ochre pit was dug near the house, which supplied fine quality pigment for many years. However, the seam was exhausted in the early years of the twentieth century and this was subsequently filled in.
Plot also suggested that less good quality ochre could be found nearby at Garsington and also at Pyrton. The ochre obtained between Ducklington and Witney (approx 10 miles west of Oxford) was employed for inferior purposes and low grade reddle8 was also found near Witney.
The small quantity of Oxford Ochre used to head this post was picked up while walking with the landowner and my great friend the pigment authority, Keith Edwards, several years ago. Sadly no evidence of larger quantities could be found.
The Decline in Production
Small amounts could still be found in the 1920s, when (echoing Plot’s account) it was suggested that one had to dig down to about 20 feet to find the seam:
Beds of highly ferruginous grit, forming the summit of the hill….6 feet
Grey sand………………………………………………………………3 feet
Ferruginous concretions……………………………………………..1 feet
Yellow sand…………………………………………………………….6 feet
Cream-coloured loam………………………………………………..4 feet
Ochre……………………………………………………………………6 inches
Beneath this there was a second bed of ochre, separated by a thin bed of clay.
In 1926, the best exposures were in small pits at the western end of the hill, and there were also small pits at the east edge of Horspath Common and on the hill south of Wheatley.
There are references to a Mr White who used to make paint for Oxfordshire wagons – mixed with linseed oil. Apparently this was manufactured at a barn near Mulberry Court, Wheatley. It was ground at nearby Wheatley windmill until the late 19th century, and was peddled around the county by packhorse.

Until the First World War the ochre that was not carted away in slabs was ground at the windmill. However, as with so many other working mills, this ceased operating at that time and it is now a private building. Some ten years ago the restoration of the windmill began and this is being carried out by the Wheatley Windmill Restoration Society, who have achieved great things as can be seen in the picture gallery on their website.
The windmill at Wheatley originally also had a kiln for burning the yellow ochre to a red, known as Burnt Oxford Ochre.

It is not surprising that for generations the buildings on the Shotover Estate have been painted in both a paint and a colour produced on the land.

Oxford Ochre was still being processed in the early twentieth century and the name appears in paint ranges of the period (see below). However the supplies were running out, for in 1928 Noel Heaton described them as being “almost exhausted”.

Shotover House
Shotover House and garden were begun in about 1714 for James Tyrell of Oakley. Tyrell died in 1718 and the house was completed by his son, General James Tyrell. There is no known record of the person who designed it, although the name of William Townesend who worked with Nicholas Hawksmoor at The Queen’s College in Oxford has been mentioned. It is also said that Sir John Vanbrugh advised the younger Tyrrell on the interior and garden layout. In 1855 the architect Joshua Sims added two wings in the same style of the original part of the house.

The garden was begun in 1718 and completed in 1730. It is a rare survivor of formal gardens of this period, laid out along an east-west axis 1,200 yards (1,100 m) long. The centrepiece of the garden east of the house is a straight canal, ending with a Gothic Revival folly. The architect of the folly is unknown, but if it was built before 1742 it may be one of the earliest examples of the Gothic Revival. The garden west of the house has a similarly long vista, ending with an octagonal temple designed in the 1730s by William Kent.
During the Second World War there was a prisoner-of-war camp in the grounds.
Please note that the house and grounds are private and are currently open by appointment only.
As with any of the colours featured on these pages, Oxford Ochre and Burnt Oxford Ochre can be mixed by Papers and Paints given short notice.
Notes
1 P.F. Tingry. Painter’s and Colourman’s Complete Guide. 1830. p.74.
2 John Smith. The Art of Painting. 1676. p.22.
3 John Smith, The Art of Painting in Oyl. 2nd edn. 1687. p.22.
4 John Pincot. Pincot’s Treatise on the Practical Part of Coach & House Painting. ca.1811. p. 31. It is possible, however, that the confusing use of the name Brown ochre by some, as a synonym for umber, might have led to this recommendation. The umbers are known for their drying ability.
5 One encounters a certain inconsistency in terminology as far as ochres are concerned. Strictly speaking the natural variety are termed ochres and the synthetic forms yellow iron oxide. However for commercial reasons they are both often referred to by the former name. It is worth remarking that the majority of the so-called ‘natural paints’ that one sees on offer are tinted with the synthetic variety! Another use of copperas to provide colour to a surface can be seen in a recent post on the analysis at Basildon Park
6 (Tingry 1830, 73).
7 Robert Plot. Natural History of Oxfordshire. 1677. p.55.
8 Reddle is a poor quality natural red ochre that was used for marking sheep. In literature it is best known in connection with Diggory Venn, the reddleman, in Thomas Hardy’s The Return of the Native.
View Larger Map













Hi Patrick,
You’ve put together a very thorough, well thought-out post here. I had never come across Oxford Ochre before. You obviously know your stuff!
best wishes, Alex.
Thanks Alex. I have been interested in Oxford Ochre for some time.